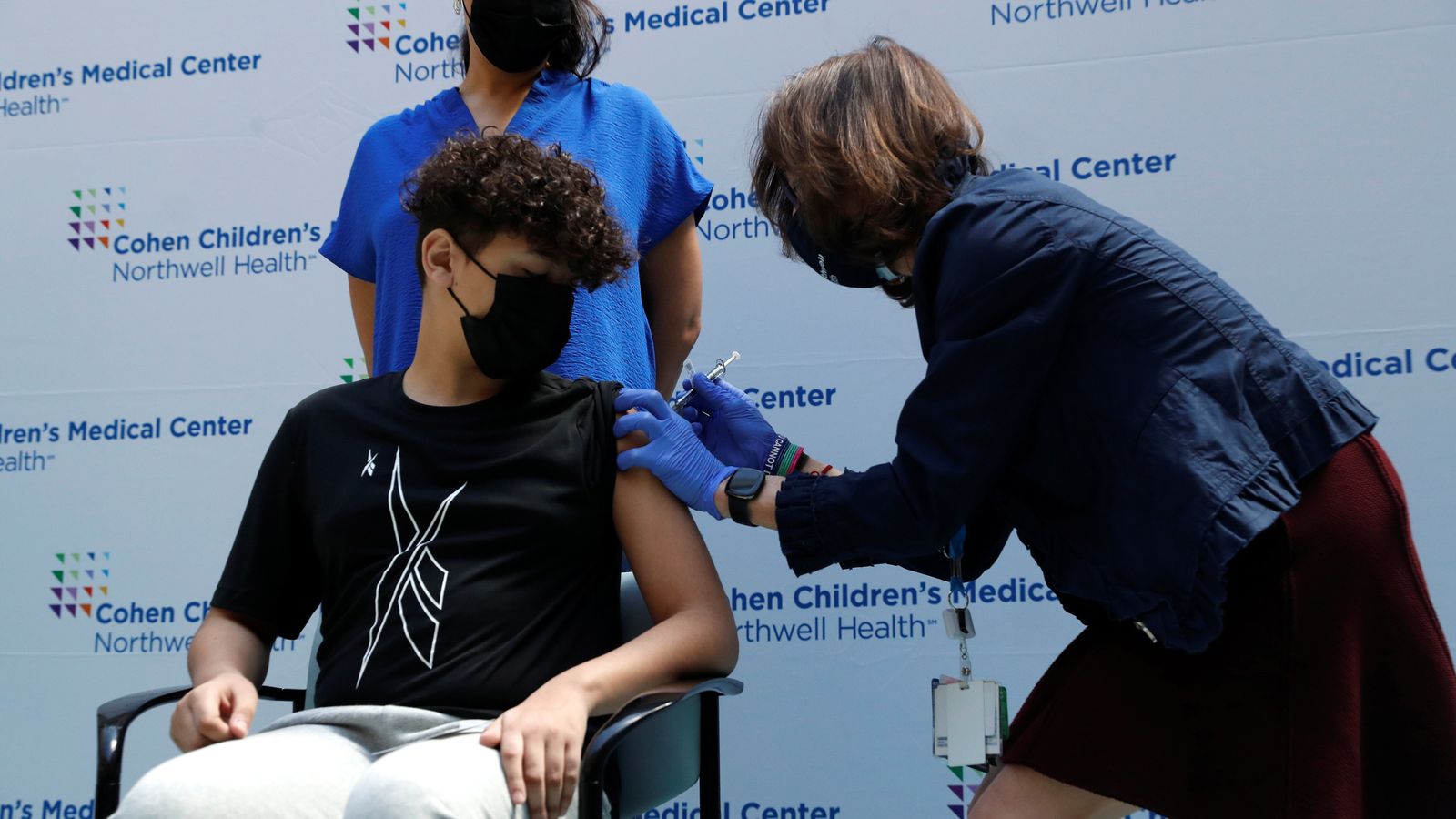
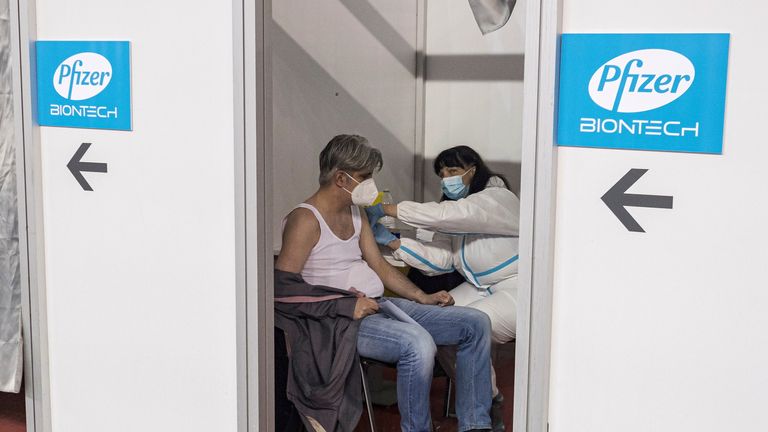
The UK’s medicines regulator has approved the Pfizer-BioNTech coronavirus vaccine for 12 to 15-year-olds.
The Medicines and Healthcare products Regulatory Agency (MHRA) said the decision followed a “rigorous review” of safety and effectiveness in that age group – and that the benefits of having the COVID jab outweighed the risks.
The Joint Committee on Vaccination and Immunisation (JCVI) will now advise whether routine vaccination should be offered to those aged 12 to 17.
Commenting on the development on Friday, Matt Hancock confirmed that the UK has “enough supply” to offer the jab to older children should the JCVI deem it “the clinically advised thing to do”.
The health secretary said: “I welcome the decision by the independent regulator that the vaccine is safe and effective for children from the age of 12 upwards.
“We’ll take clinical advice from the Joint Committee on Vaccination and Immunisation on how that should be taken forward, and then we’ll follow that clinical advice.
“We have enough supply to be able to vaccinate children should that be the clinically advised thing to do.
“We now have a vaccine that’s approved for the use of children aged 12 and over, but I want to make sure this is all done following clinical advice, to make sure that we get this country out of this pandemic as safely as we can.”
Dr June Raine, MHRA chief executive, said: “We have in place a comprehensive safety surveillance strategy for monitoring the safety of all UK-approved COVID-19 vaccines and this surveillance will include the 12- to 15-year age group.”
The Department for Health and Social Care added that it will continue to be “guided by the expert advisors”.
The Pfizer/BioNTech jab was approved for use in the UK for 16 and 17-year-olds in December 2020.
The JCVI’s current advice is that those aged 16 to 18 should be offered vaccination if they are in a priority Phase 1 group or they are the household contacts of someone who is immunosuppressed.
There is no routine vaccination of under 18s currently under way.
More than 2,000 children were involved in the clinical trial to determine the safety of the Pfizer/BioNTech vaccine, the chairman of the Commission on Human Medicines said.
Professor Sir Munir Pirmohamed said based on the data, the vaccine’s benefits “do outweigh any risk”.
He added out of the 2,000 children studied as part of the randomised, placebo-controlled clinical trials, there were no cases of COVID-19 from seven days after the second dose, compared with 16 cases in the placebo group.
In addition, data on neutralising antibodies showed the vaccine working at the same level as seen in adults aged 16-25 years.
“These are extremely positive results,” he said.
Meanwhile, Mr Hancock reiterated that it is “very early” to deem whether the 21 June lockdown easing date will go ahead.
The health secretary said: “It’s too early to make a decision about the step that will happen not before the 21 June – we’re looking at the data every single day, and we’ll make a decision and publish it later in this month.”
He added: “The most important data that we’re looking for ahead of making the decision about whether we can go ahead with step 4 on the 21 June is how effective the vaccine has been in breaking the link from cases to hospitalisations and deaths.
“We know that the vaccine works and has reduced by a long long margin your likelihood of ending up in hospital or even worse of dying from COVID, we know that it’s effective – we want to see in the real world data just how effective it is.
“Because we’ve seen the number of cases go up – we always expected that to happen – and we’ve not seen a rise in the number of people going to hospital in the same way.
“And we just want to see how effective the vaccine is at stopping what was an inevitable link from the increase in cases to the increase in hospitalisations.
“We’re looking very very carefully at the data and we’ll say more when we know more.”